Three Physical Properties Aldehydes
Three Physical Properties Aldehydes. The physical properties of some aldehydes and ketones are given in table 27.1. Methanal, the first member of the series in aldehyde, is a gas at room temperature, while ethanal is a volatile liquid.

 3B 4.8 Physical Properties of Aldehydes & Ketones YouTube from www.youtube.com
3B 4.8 Physical Properties of Aldehydes & Ketones YouTube from www.youtube.comWater solubility (k) (k) (%) methanal 181 252 55 ethanal 150 294 f propanal 192 322 20 Ethanal is a volatile liquid. Physical properties of aldehydes and ketones.
Water solubility decreases as the size of the molecule increases. What are the 3 physical properties of aldehydes and ketones?
The physical properties of aldehydes and ketones are described as follows. 21.3 physical properties of aldehydes and ketones.

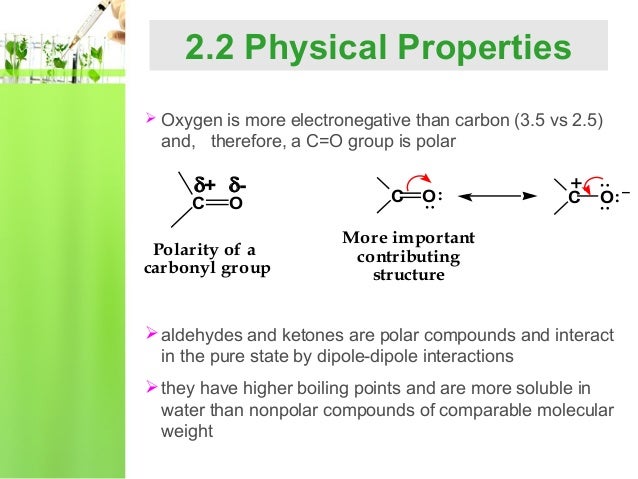
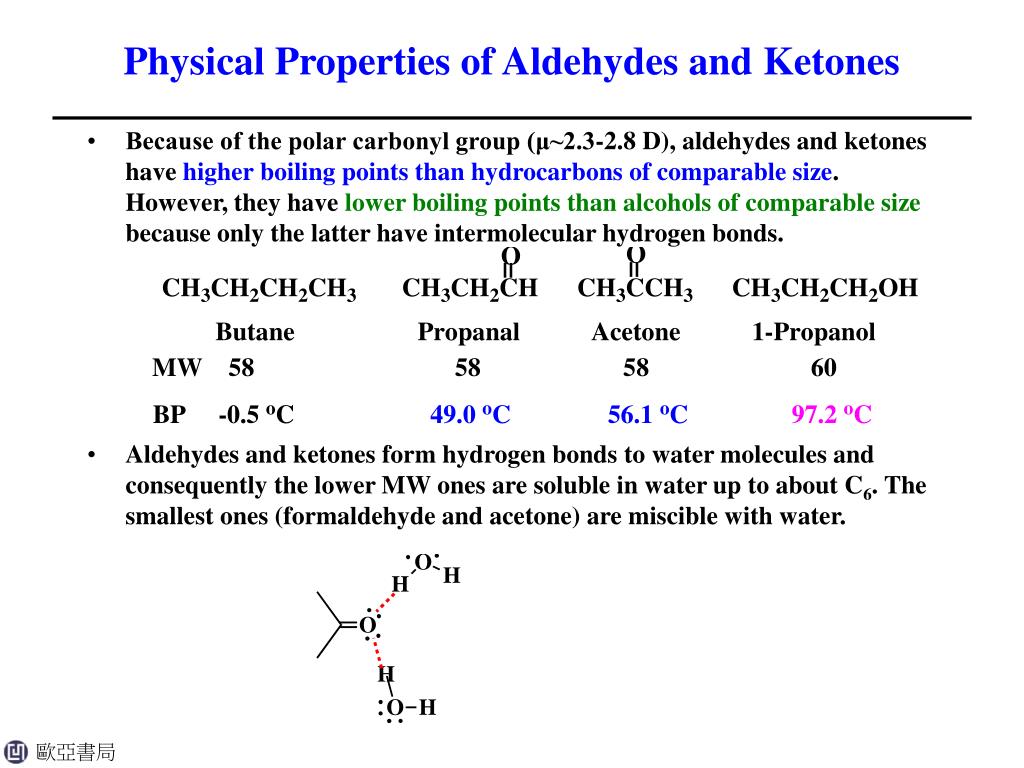
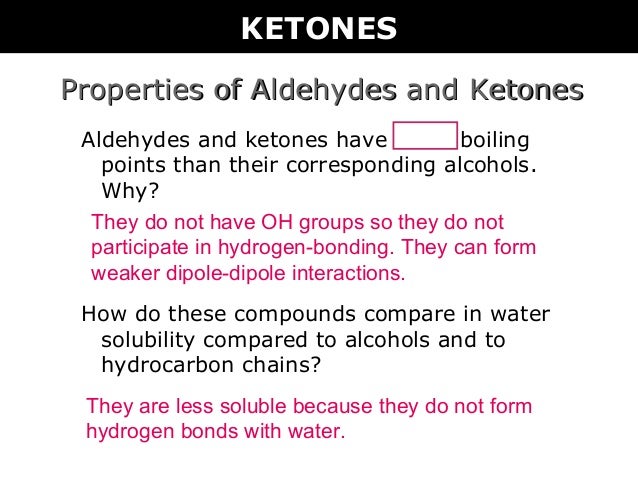
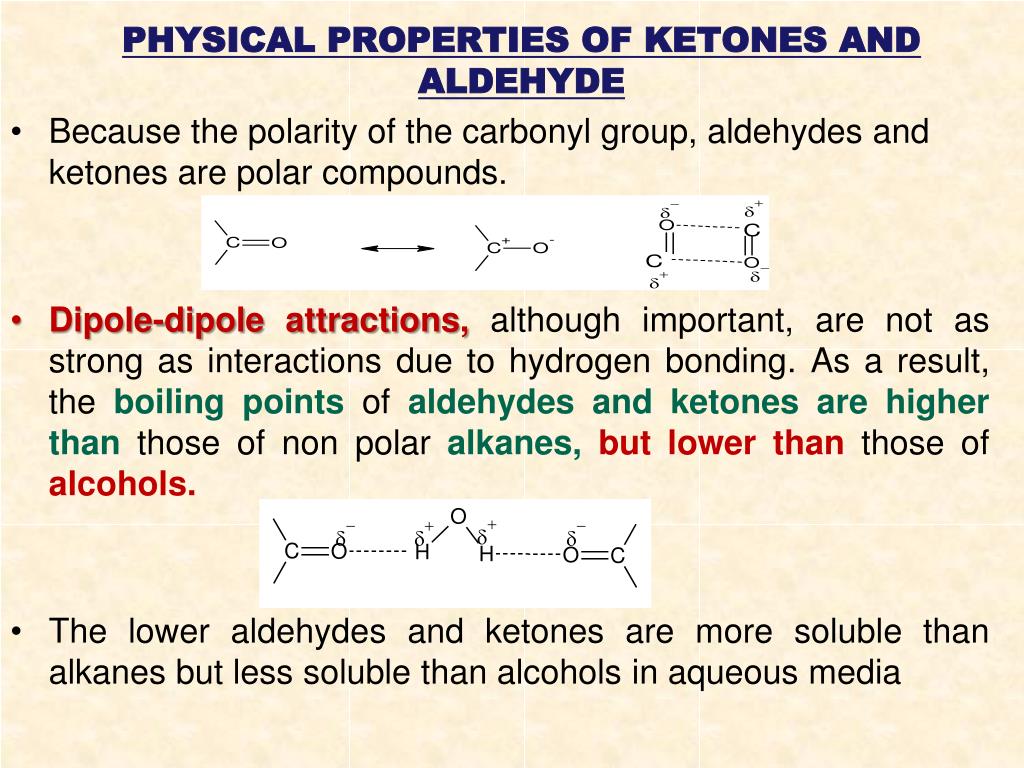
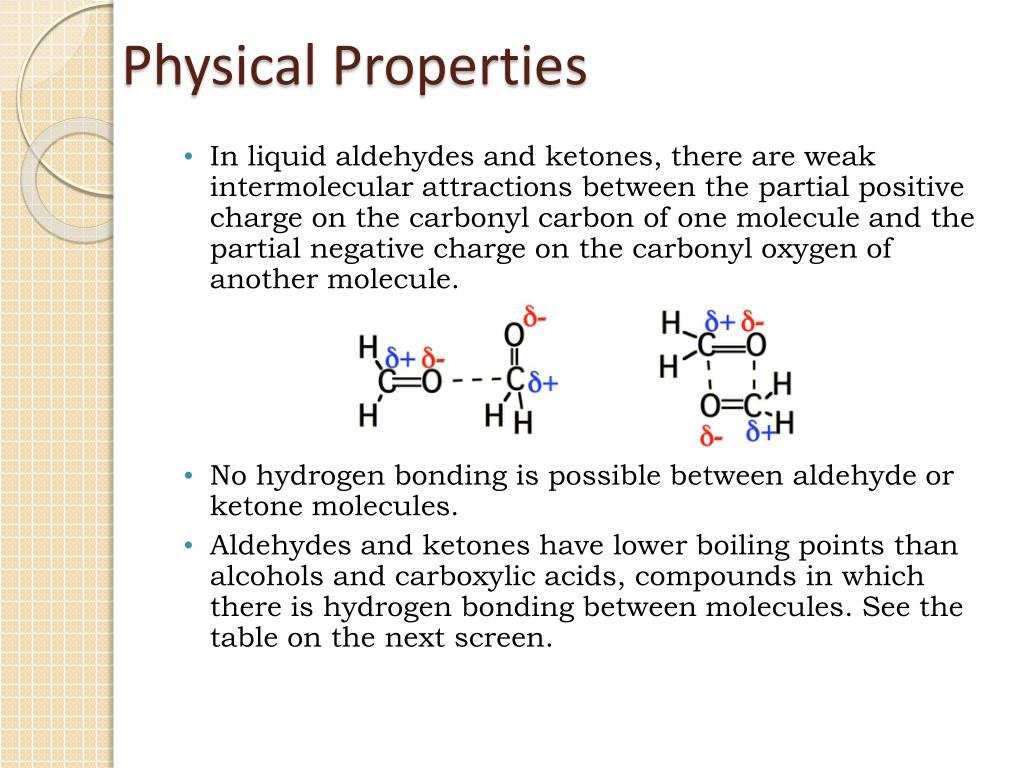
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. The aldehyde produced can be oxidised further to a carboxylic acid by the acidified potassium dichromate(vi) solution used as the oxidising agent.

Similarly, other aldehydes and ketones are either gas or liquid at room temperature. Cheméo is only indexing the data, follow the source links to retrieve the latest data.

The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. Before dealing with the chemical properties of aldehydes and ketones, it is worth considering their physical features.
The aldehyde produced can be oxidised further to a carboxylic acid by the acidified potassium dichromate(vi) solution used as the oxidising agent. Other aldehydes and ketones are liquid or solid at room temperature.

Hydrogen bonds are formed between the oxygen atoms and also between the hydrogen atom of water and also between the hydrogen atom of the aldehyde and the oxygen atom of water. Moreover, all other aldehydes and ketones.

The physical properties of some aldehydes and ketones are given in table 27.1. Lower aldehydes and ketones are volatile in nature.
Similarly, other aldehydes and ketones are either gas or liquid at room temperature. The physical properties of aldehydes and ketones are:
Other aldehydes and ketones are liquid or solid at room temperature. What are the 3 physical properties of aldehydes and ketones?

The reactivity of these compounds arises largely through two features of their structures: C alcohols, ketones and aldehydes, alkanes.

Other aldehydes and ketones are liquid or solid at room temperature. Similarly, other aldehydes and ketones are either gas or liquid at room temperature.

What are the 3 physical properties of aldehydes and ketones? Physical properties of aldehydes and ketones.
The lower members of this class dissolve perfectly in h 2 o and interact well with organic solvents. The source is also providing more information like the publication year, authors and more.

The carbonyl group is present in both aldehydes and ketones compounds. Methanal is a gas at room temperature.
Aldehydes are polar molecules, and many reagents seek atoms with a deficiency of electrons. Water solubility decreases as the size of the molecule increases.

R c r' o h o r' c r o h Aldehydes have hydrogen atom on the carbonyl group that can be easily converted to the hydroxyl group, so the aldehydes are easily oxidized to the carboxylic acids.

In each of the functional groups below, what is the strongest intermolecular force in each? The carbonyl group is present in both aldehydes and ketones compounds.

The source is also providing more information like the publication year, authors and more. The physical properties of aldehydes and ketones are as below.

In each of the functional groups below, what is the strongest intermolecular force in each? C alcohols, ketones and aldehydes, alkanes.
At Room Temperature, Methanol Behaves As A Gas Whereas Ethanol Is In Liquid Form That Is Volatile In Nature.In order of highest boiling point to lowest for alcohols ketones, alkanes, and aldehydes. Before dealing with the chemical properties of aldehydes and ketones, it is worth considering their physical features. The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Physical Properties Of Aldehydes & Ketones 1) Boiling Point.Physical properties of aldehydes and ketones. In each of the functional groups below, what is the strongest intermolecular force in each? The physical properties of aldehydes and ketones are:
Similarly, Other Aldehydes And Ketones Are Either Gas Or Liquid At Room Temperature.C alcohols, ketones and aldehydes, alkanes. So let's look at solubility next. The carbonyl group is present in both aldehydes and ketones compounds.
The Ketones Are Mostly Liquids Or Solid At Room Temperature.Water solubility decreases as the size of the molecule increases. Ethanal is a volatile liquid. − boiling points of aldehydes and ketones.
Physical Properties Of Aldehydes And Ketones.The physical properties of aldehydes and ketones are as below. Ketones are low melting solids or volatile liquids. The lower members of this class dissolve perfectly in h 2 o and interact well with organic solvents.
Komentar
Posting Komentar